Online Course
Unlocking Regulatory Success for Digital Health Product Developers
A good regulatory strategy is essential to a good business strategy.

I wish this course existed when we were getting started 10 years ago!

Course overview
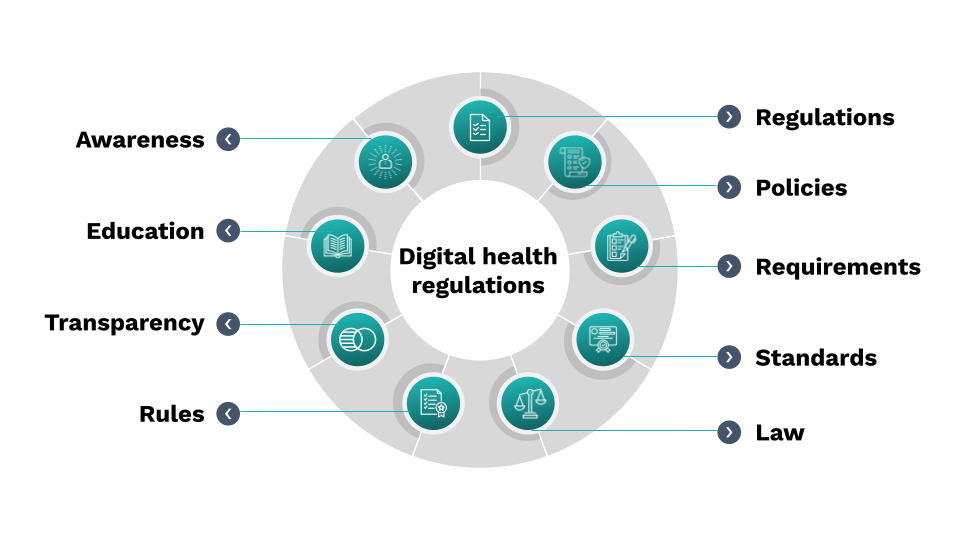
It’s difficult for digital innovators to know how to interact with regulators, which ones, and when. This course will remove the mystery. Learn how to build a fit-for-purpose regulatory strategy that strengthens your business strategy and differentiates your product in today’s digital health market.
Learning outcomes
Identify whether digital health products in your portfolio are likely to be regulated and through which mechanisms. Build and implement a regulatory strategy that advances the success of your products. Build evidence based, high-quality products without paying for expensive consulting services.
Discover why good regulatory strategy is essential to a good business strategy.
Topics Include
-
Today’s Regulatory Landscape
What to know about the current and evolving landscape of digital health product regulation by FDA.
-
Elements of a Robust Strategy
Details on the components of a fit-for-purpose regulatory strategy and where to begin when building one.
-
Ensuring Successful Implementation
Obtain the skills necessary to implement your regulatory strategy and interact with regulators.
Meet the instructors
Learn from DiMe experts and world class guest faculty – including legal, strategy, and security experts from industry, academia, and regulatory bodies – at the cutting edge of the most difficult ethical questions in digital health.
Reviews
Read what our learners are saying…

I work for multiple health startups now and all of them play in a regulated space but very few have a grasp of the regulations that govern their businesses. I’ve found tremendous value in being the guy who can bridge business, healthcare, and law/reg and this course has helped cement some of the regulatory concepts I struggled with in digital health as well as gives me a cool certification to put some weight behind my experience without having “regulatory” on my resume.

Learning from industry experts throughout this course was very impactful. The course helped me appreciate the challenges and successes of digital innovation using real world cases and provided an understanding of available toolkits to thrive in this volatile and uncertain landscape. I really appreciated the parts of the course that emphasized the need for partnership in development at every stage and the quality improvement tips provided.

The course provided a simple and efficient approach to developing a general understanding of the regulatory frameworks for digital health products–with links and references to dive deeper as needed.

I think the regulatory chart/table for the different device categories is extremely helpful. I also think the outline for developing a fit-for-purpose regulatory strategy provides key guidance to unify the development of trusted digital health products.

The emphasis for a fit for purpose reg strat is practical and not just a theoretical concept that I plan to back to work.

Despite being one of the instructors in the course, I definitely learned one or two new things. However, maybe more importantly, the well thought through course structure really lends itself to seeing the bigger picture of digital health product regulation and how to use it to frame product development.

With the knowledge gained from this course I will now be able to confidently guide my digital health clients on every stage of developing and implementing a robust regulatory strategy.

Amazing content overall!

Working daily with med-tech start-ups this course will help us guide them through regulatory challenges, defining a pathway to move forward.

One of my clients has a new clinical research program and is open to innovation. We will develop technologies that reach patients and clinicians, and we will need to work closely with the FDA. I will return to this course to refresh on needed actions and take advantage of the organized, linked external resources and explanations. This course also provides the evidence we need to justify early and often communication with the FDA.
Earn a Credly Badge
Once you complete a course, you’ll receive digital credentials to showcase your professional development in the digital healthcare field.
-
Share your proof of achievements on social media
-
Provides recognition for acquiring new skills
-

Allows hiring managers to easily see acquired competencies

More courses coming soon
The Digital Medicine Academy®️ is committed to offering valuable lessons in pace with the evolving digital healthcare field. Check back for new course offerings.