Introducing V3+: Extending DiMe’s V3 Framework to add usability validation
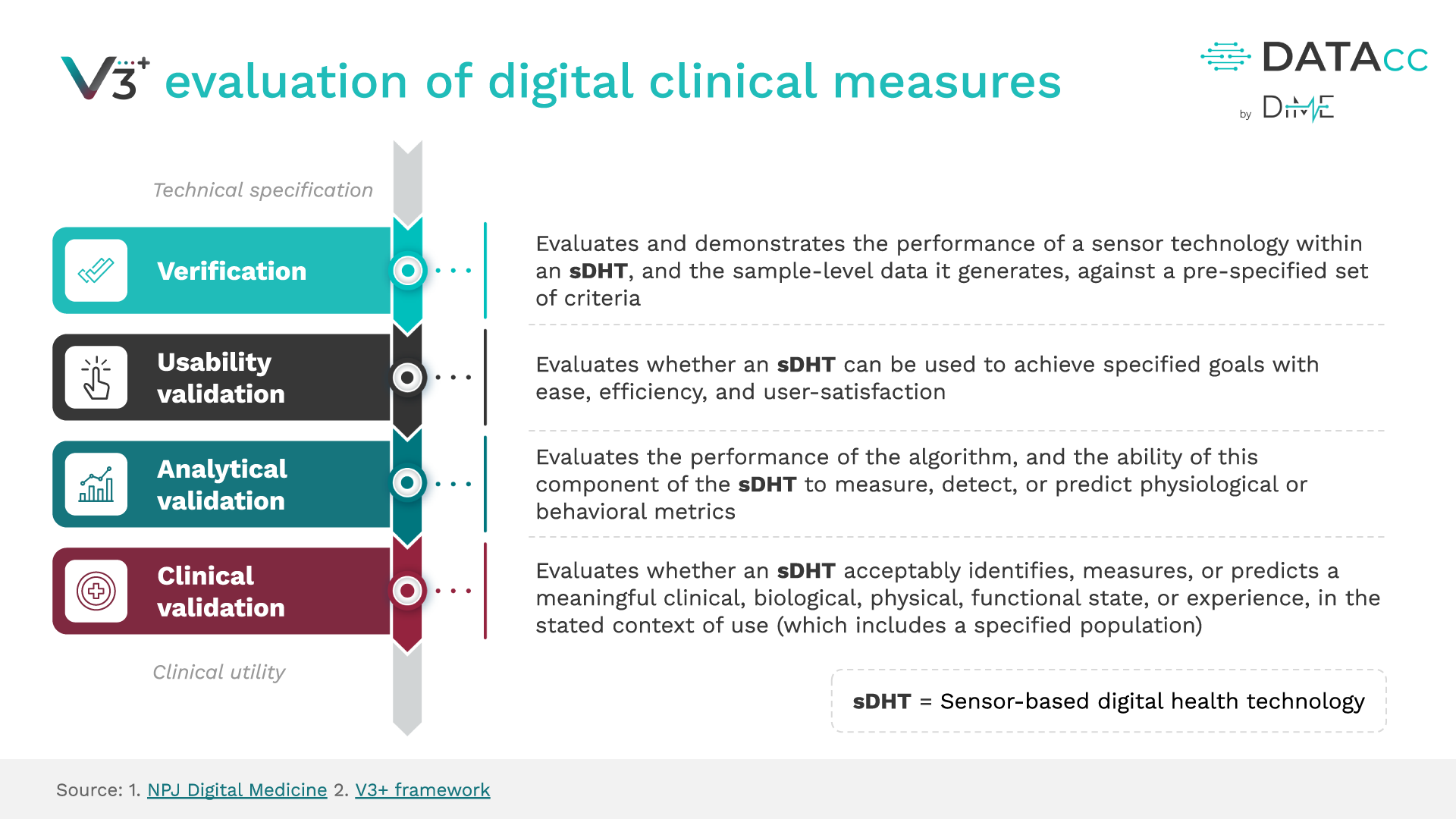
The Digital Health Measurement Collaborative Community (DATAcc) by the Digital Medicine Society (DiMe) has extended DiMe’s Verification, Analytical Validation, and Clinical Validation (V3) Framework with V3+ to include a fourth component – usability validation.
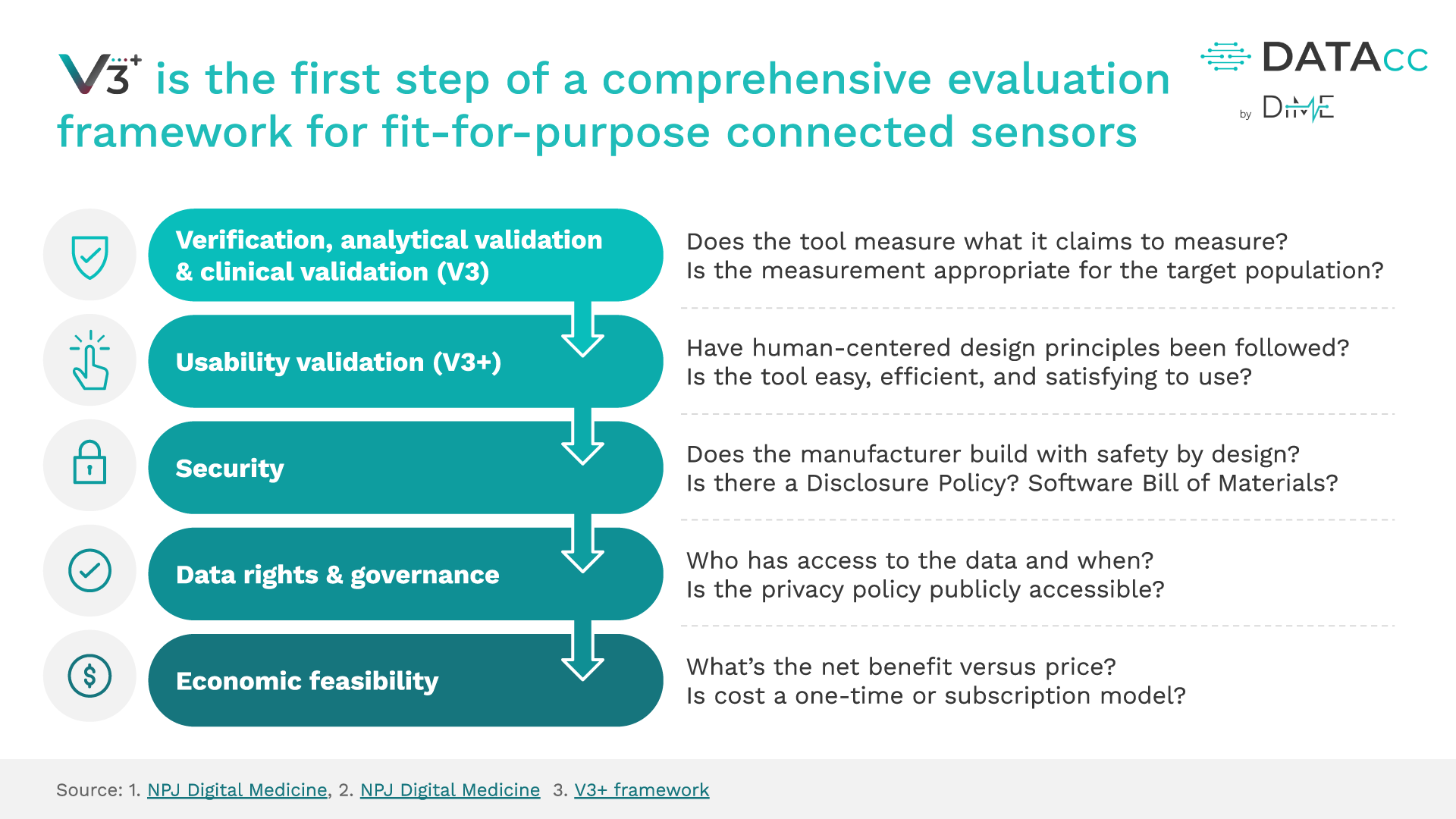
The addition of usability validation as an equally important step in establishing whether sensor-based digital health technologies (sDHTs) are fit for purpose is an essential extension of V3 to help developers keep pace with the deployment of digital clinical measurement at scale.
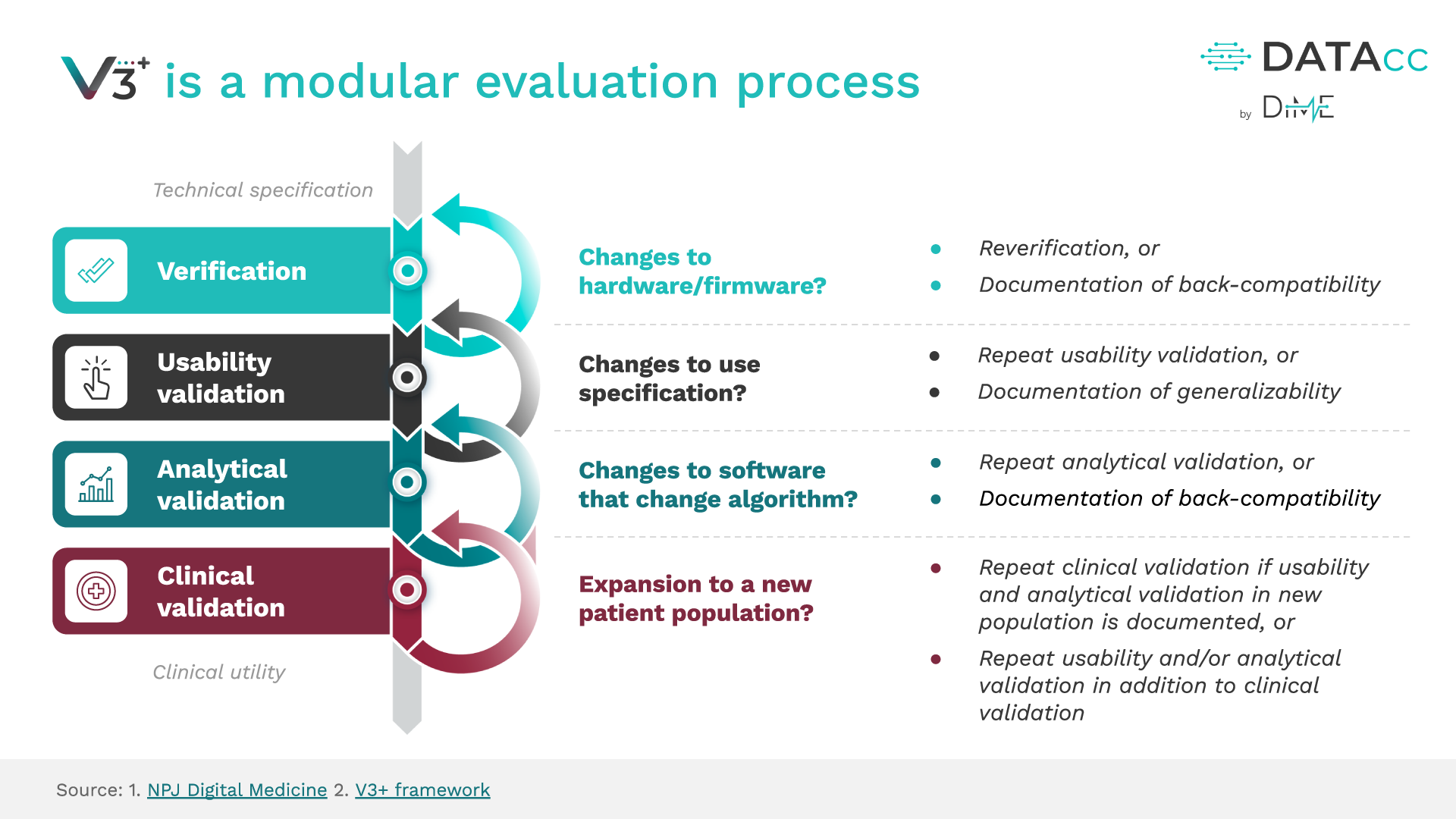
The original V3 Framework is wholly encapsulated and unchanged within V3+ – combining the power of V3 with new user-centric components. Using V3+ and its resources will enable you to build high-quality products, transform the way we care for people, and conduct clinical trials.
Project partners
We were proud to define best practices for usability validation and extend the V3 Framework to V3+ with the following partners.












We remain grateful to the experts who defined the original V3 Framework, which remains the industry standard for sDHT evaluation. Jennifer C. Goldsack, Andrea Coravos, Jessie P. Bakker, Brinnae Bent, Ariel V. Dowling, Cheryl Fitzer-Attas, Alan Godfrey, Job G. Godino, Ninad Gujar, Elena Izmailova, Christine Manta, Barry Peterson, Benjamin Vandendriessche, William A. Wood, Ke Will Wang & Jessilyn Dunn.
Get involved
Join our next project to develop a value framework, industry benchmarking tools, forecasting models, and a modular template business case framework to help innovators invest in digital endpoints and advance their broad adoption and scale in clinical research.

V3: The de facto standard across the industry
Since its publication in 2020, DiMe’s original V3 Framework has emerged as the go-to international resource for evaluating whether sDHTs are fit for purpose.
Today, it maintains successful momentum; it has been accessed over 30,000 times, cited more than 250 times in peer-reviewed journals, and leveraged by over 140 teams, including NIH, FDA, and EMA. V3+ is an extension that meets the field’s evolving needs and is poised to gain widespread adoption and become the new, expanded industry standard, just as V3 has been.